Abstract
We recently reported that CD99 is highly expressed on disease initiating stem cells in myeloid malignancies. In acute myeloid leukemia (AML), high expression of CD99 identified human leukemia stem cells (LSCs) with enhanced leukemia-initiating capacity and gene signatures associated with hematopoietic stem cell (HSC) self-renewal. LSCs with high CD99 expression were also marked by significant depletion of ribosomal protein transcripts, and we have subsequently confirmed that they exhibit reduced levels of protein translation as measured by polysome profiling.
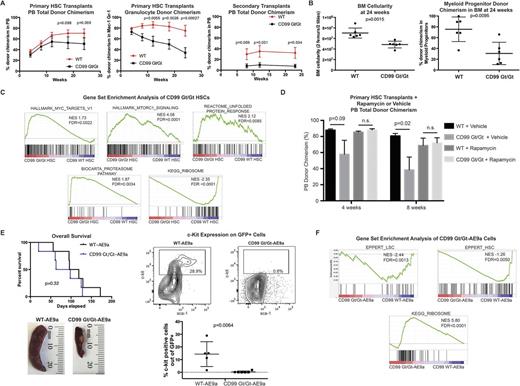
To elucidate the mechanisms by which CD99 promotes self-renewal in hematopoietic cells, we characterized CD99-deficient mice generated by gene trapping (B6- CD99Gt(pU-21T)44lmeg , or CD99 Gt/Gt). At steady-state, homozygous CD99 Gt/Gt mice exhibit normal CBCs, bone marrow (BM) cellularity, and hematopoietic stem and progenitor frequencies. To test the function of CD99 Gt/Gt HSCs, we FACS-purified HSCs (Lin-Sca-1+c-Kit+CD34-CD150+) from CD99 Gt/Gt and wild-type (WT) mice and transplanted them into lethally irradiated WT recipients. At early time points, CD99 Gt/Gt HSCs and WT HSCs engrafted equally well. However, over the course of 24 weeks, there emerged in mice transplanted with CD99 Gt/Gt HSCs a strong trend towards decreased peripheral blood (PB) donor chimerism, as well as a progressive and highly significant decline in granulocyte (Mac-1+Gr-1+) donor chimerism, suggesting that CD99 Gt/Gt HSCs may gradually exhaust after transplantation (Fig.1A). BM evaluation at 24 weeks revealed no differences in HSC donor chimerism, but there was a significant decrease in myeloid progenitor (Lin-Sca-1-c-Kit+) donor chimerism and BM cellularity in mice transplanted with CD99 Gt/Gt HSCs (Fig.1B), consistent with decreased HSC functional output. In non-competitive secondary transplants using unfractionated BM, CD99 Gt/Gt HSCs exhibited a marked decrease in engraftment, consistent with impaired self-renewal.
To assess the impact of CD99-deficiency on transcriptional output, we performed RNA-seq on HSCs from CD99 Gt/Gt and WT mice, identifying 327 differentially expressed genes (p<0.01). Gene set enrichment analysis identified an increase in transcripts associated with the proteasome, mTOR signaling, and c-MYC targets, as well as depletion of ribosomal protein transcripts (Fig.1C). This gene expression pattern suggests that CD99 Gt/Gt HSCs exhibit proteotoxic stress, which has been shown to promote transcriptional repression of ribosomal proteins.
Given that tightly regulated translation has been shown to be essential for HSC function, as well as our findings in HSCs and LSCs that suggest that CD99 negatively regulates translation, we hypothesized that CD99 promotes self-renewal in HSCs by limiting protein translation. To test this, we transplanted HSCs from CD99 Gt/Gt and WT mice as before and treated recipient mice with the mTOR inhibitor rapamycin (4 mg/kg daily). Rapamycin completely rescued the engraftment defect of CD99 Gt/Gt HSCs at eight weeks (Fig.1D), strongly suggesting that their functional defects are mediated by increased translation.
Finally, we measured CD99 expression on leukemia cells from mouse AML models including MLL-AF9 , AML-ETO9a , and NUP98-HOXD13, findingCD99 to be overexpressed compared with normal myeloid progenitors (Lin-Sca-1-c-Kit+), similar to our prior observations in human AML. Because AML1-ETO is correlated with high CD99 mRNA expression in human AML (ECOG 1900 cohort), we tested whether CD99 is required for AML1-ETO induced leukemogenesis. c-Kit+ cells from CD99 Gt/Gt and WT mice were transduced with AML1-ETO 9a (AE9a) and transplanted into lethally irradiated recipients. While there was no significant difference in survival between mice transplanted with CD99 Gt/Gt-AE9a vs. WT-AE9a cells (81 d vs. 108 d, p=0.32), CD99 Gt/Gt-AE9a grafts exhibited less splenomegaly and a dramatic reduction in c-Kit+ cells (43-fold, p=0.0064), suggestive of increased differentiation of leukemia cells and depletion of LSCs (Fig.1E). Consistent with this, RNA-seq revealed in CD99 Gt/Gt-AE9a cells depletion of HSC/LSC-associated gene signatures, as well as enrichment for ribosomal protein transcripts (Fig.1F).
Together, these findings reveal an essential role for CD99 in HSC and LSC function, suggesting that it promotes self-renewal in part by acting as a key regulator of protein translation.
Levine: Roche: Research Funding; Roche: Research Funding; Celgene: Research Funding; Qiagen: Equity Ownership; Qiagen: Equity Ownership; Celgene: Research Funding. Park: Forty Seven, Inc.: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.